900 results found | searching for "developed"
-
Software Product Engineering and Development Solutions https://www.emorphis.com/software-product-development-company-service-provider/ We understand how important software product engineering process is for product companies because it's not only your product idea that award you success, but the right idea developed in a right way is the key to success.
-
The global Bioprocess Bags Market was valued at USD 3.50 billion in 2023 and is projected to reach USD 13.78 billion by 2032, growing at an impressive CAGR of 16.46% between 2024 and 2032. This remarkable growth reflects the surging adoption of single-use technologies across the biopharmaceutical sector, where efficiency, scalability, and contamination control have become essential for both research and large-scale drug production. Bioprocess bags are critical tools for storage, mixing, and transport of biopharmaceutical fluids, offering superior sterility and reducing the risks associated with traditional stainless-steel systems. Their increasing integration into upstream and downstream processing highlights a transformative shift in the way biologics, vaccines, and cell-based therapies are developed and manufactured. Market Dynamics: Why the Industry Is Scaling at Unprecedented Levels The rapid expansion of biologics and biosimilars pipelines worldwide is a major force fueling the demand for bioprocess bags. As pharmaceutical companies face heightened pressure to bring therapies to market quickly, flexible and reliable solutions like bioprocess bags offer significant advantages. They lower capital costs, reduce cleaning validation requirements, and minimize the risk of cross-contamination. Furthermore, the COVID-19 pandemic accelerated the acceptance of single-use technologies. Manufacturers worldwide experienced firsthand the flexibility these systems provided in ramping up vaccine production. That momentum continues to shape bioprocessing strategies, with companies investing heavily in disposable solutions to ensure agility and speed. Bioprocess bags are not just limited to large-scale manufacturing. They are increasingly being adopted in academic research labs, contract development and manufacturing organizations (CDMOs), and emerging biotech startups, where scalability and cost-effectiveness are equally critical. Technological Advancements Enhancing Market Potential The bioprocess bags industry is witnessing significant innovation in materials, design, and performance. Leading manufacturers are focusing on developing multilayer films that provide enhanced durability, high oxygen barrier properties, and compatibility with a wide range of biologic materials. Additionally, bags are being designed with advanced monitoring systems that integrate sensors to track pH, dissolved oxygen, and other key parameters in real time. These smart bag solutions align with the biopharma industry’s push toward process intensification and continuous manufacturing. The development of customizable and scalable bag formats is further supporting small- and mid-sized biotech firms that require flexibility without compromising compliance with regulatory standards. Regional Outlook: North America and Asia-Pacific at the Forefront North America continues to lead the bioprocess bags market, driven by the strong presence of biopharmaceutical giants, advanced research infrastructure, and favorable regulatory frameworks. The region’s focus on biologics, particularly monoclonal antibodies and gene therapies, sustains robust demand for single-use solutions. Meanwhile, Asia-Pacific is emerging as a hotspot for growth. Rapidly expanding biotech clusters in countries like China, India, and South Korea are attracting global investments. Governments in the region are promoting domestic biologics production, further boosting adoption of bioprocess bags. The lower cost of production combined with strong demand for biosimilars positions Asia-Pacific as a key growth engine for the forecast period. Key Market Drivers Booming Biologics and Biosimilars Market: Rising prevalence of chronic diseases and demand for advanced therapies have placed biologics at the center of global healthcare, propelling the need for reliable bioprocessing solutions. Shift Toward Single-Use Technologies: The move away from stainless-steel systems to disposable bags significantly reduces downtime, contamination risks, and operational costs. Growing Investment in Cell and Gene Therapy: Breakthroughs in regenerative medicine demand flexible and sterile solutions that bioprocess bags are uniquely designed to provide. Rapid Expansion of Contract Manufacturing Organizations: As CDMOs scale operations globally, the reliance on single-use technologies becomes indispensable. Competitive Landscape The bioprocess bags market is highly competitive with a mix of global leaders and specialized niche players. Companies are focusing on collaborations, acquisitions, and product launches to strengthen their portfolios. Leading players are also investing in expanding production capacities to meet the surging global demand. Recent trends show a rise in partnerships between suppliers and CDMOs to co-develop customized solutions. Such collaborations enable end-users to achieve process efficiencies while ensuring compliance with regulatory standards. Challenges to Watch Despite its strong trajectory, the market does face hurdles. Concerns related to leachables and extractables from plastic materials remain under scrutiny, particularly from regulatory authorities. Additionally, supply chain disruptions for raw materials can pose risks to production continuity. However, industry stakeholders are addressing these challenges through rigorous testing protocols, improved material science, and diversification of supply chains to ensure consistent availability of high-quality bioprocess bags. Future Outlook The bioprocess bags market is positioned for exceptional growth throughout the next decade. The convergence of biologics expansion, single-use adoption, and smart technology integration sets the stage for continued innovation. With advancements in material engineering and automation, bioprocess bags are expected to evolve from simple storage tools to highly sophisticated components that actively support biomanufacturing. As the healthcare industry shifts toward precision medicine, biologics and cell-based therapies will demand even greater flexibility and sterility in manufacturing processes. Bioprocess bags are uniquely aligned to meet these evolving needs, cementing their role as a cornerstone of modern bioprocessing. Industry analysts predict that the next phase of growth will be marked by hybrid systems, where single-use technologies like bioprocess bags coexist with stainless-steel infrastructure to optimize performance, scalability, and sustainability. Conclusion With a projected market value of USD 13.78 billion by 2032, the bioprocess bags industry is set to reshape the global biopharmaceutical manufacturing landscape. Its rapid adoption underscores the industry’s commitment to efficiency, sterility, and adaptability in an increasingly competitive market environment. For stakeholders across the value chain, from biotech startups to global pharmaceutical leaders, investing in bioprocess bag solutions represents not just an operational advantage but a strategic imperative. Read More: https://www.snsinsider.com/reports/bioprocess-bags-market-6853
-
ISO/IEC 42001 is the first international standard for Artificial Intelligence Management Systems (AIMS). It provides a framework for organizations to ensure that AI is developed and used in a safe, ethical, transparent, and accountable manner. Builds trust in AI systems. Ensures ethical and transparent use. Manages risks like bias and misuse. Learn more: https://sqccertification.com/iso-42001-certification-requirement/ Visit Site: https://sqccertification.com/ Call Us: +91-9990747758 Location: https://g.co/kgs/aJsxrjf #iso #isoindia #isostandard #sqccertification #iso/iec 42001 #artificial intelligence
-
The Economics of AI in Healthcare, ROI Models That Work https://emorphis.health/blogs/economics-of-ai-in-healthcare-roi-models/ Many organizations have tested AI pilots, but few have developed a clear understanding of return on investment (ROI). Without a strong financial case, it is difficult for hospitals, payers, or life sciences companies to scale AI adoption. This is where the economics of AI in healthcare becomes a critical conversation.
-
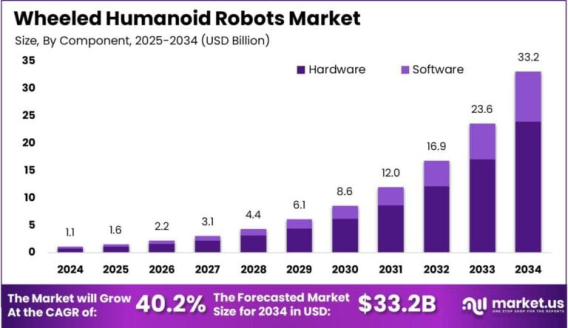
Wheeled Humanoid Robots Market size is growing at a CAGR of 40.20% The Global Wheeled Humanoid Robots Market size is expected to be worth around USD 33.2 Billion By 2034, from USD 1.13 Billion in 2024, growing at a CAGR of 40.20% during the forecast period from 2025 to 2034. In 2024, North America led the wheeled humanoid robots market with a 43.2% share and USD 0.4 billion in revenues. The U.S. market was valued at USD 0.44 billion and is growing rapidly, with an estimated CAGR of 38.7%. Read more - https://market.us/report/wheeled-humanoid-robots-market/ Wheeled humanoid robots are advanced robotic systems designed with a humanoid body structure but move using wheels instead of legs. These robots are typically developed for applications requiring both human-like interaction and efficient locomotion in indoor and semi-structured environments. Their ability to communicate, perform service tasks, and navigate smoothly makes them suitable for customer service, healthcare, logistics, and personal assistance. Their wheeled design simplifies balance and speed control, making them more practical than bipedal robots in several use cases. The wheeled humanoid robots market is witnessing steady growth, driven by rising demand in sectors like retail, hospitality, and eldercare. With industries increasingly looking to automate tasks involving human interaction, the market is expected to grow as both startups and major players invest in robotic solutions. Asia-Pacific and North America remain key regions due to their strong robotics manufacturing base and growing consumer service sectors. Government-led tech initiatives and private sector R&D efforts are also accelerating development and deployment across industries. One of the main driving forces is the rapid evolution of AI and machine learning, which enables these robots to interpret human behavior and adapt accordingly. Growth in urban automation, labor shortages in service sectors, and the need for contactless operations post-pandemic are further fueling market adoption. As people become more comfortable with service robotics in everyday settings, the market is benefitting from increased public acceptance and commercial viability.
-
Benefits of ISO 50001 Certification Energy plays an important role in the operation of any business. From machines in factories to lights in offices, it is used everywhere. However, with rising energy costs and increasing environmental concerns, it has become essential for organisations to manage energy usage more responsibly. That’s where ISO 50001 Certification comes in. It’s a global standard that helps businesses manage and save energy smartly. Getting ISO 50001 Certified shows that a company is serious about saving energy, cutting waste, and working more efficiently. Understanding the ISO 50001 Standard ISO 50001 is a globally recognised standard developed by ISO to help companies build a system for managing energy, known as an Energy Management System(EnMS). The goal of ISO 50001 is to help companies use less energy, improve how they use it, and save money. It follows a simple cycle: Plan how to save energy. Do what you planned. Check if it worked or not. Act to make it better. This is called the PDCA cycle (Plan-Do-Check-Act), and it helps companies keep improving over time. And, the best part is — you don’t need to buy new machines. ISO 50001 does not force you to spend money. Instead, it helps you find small changes in your daily work that can save energy. It guides you to make better decisions, step by step. Impact on Business Operations Implementing ISO 50001 helps an organisation to manage its energy usage more effectively, leading to improvements in daily operations. For example, the company may choose machines that use less power. It may also fix old machines more often to avoid energy loss. Workers may get training to save energy in small tasks. Reports are made to track where energy is used and how much is being used. This increased awareness of energy usage within the company helps save money and makes work smoother. Top Benefits of ISO 50001 Certification Some of the main benefits of ISO 50001 are as follows: 1. Lowers Energy Cost: When you use energy better, you spend less money. 2. Less Harm to the Environment: Using less energy means less pollution and fewer carbon gases. 3. Better Workflow: You check your work often and fix problems quickly, which improves work efficiency. 4. Follow Government Rules: ISO 50001 helps you meet energy laws more easily. 5. Build Brand Reputation: People trust companies that care about saving energy. This builds your brand image. Who Should Get ISO 50001 Certified? Any company that uses energy can use ISO 50001. It is for both small and large businesses. If you use energy for lights, machines, air conditioning, or heating, this certification can help you. Some examples include: Factories. Power stations. Food companies. Hospitals. Schools and colleges. IT companies. Building and real estate firms. Why Choose Us? At SQC, we bring hands-on experience to help businesses meet ISO 50001 requirements smoothly and efficiently. Our process is simple, transparent, and built around your needs. Our strengths are: Experienced auditors. Specific guidance based on the goals, size, and structure of your company. Accredited certification to increase your reputation. Contact us Apply Now- https://forms.gle/LWSsNAqyDbW38eU76 Visit our website- www.sqccertification.com Call us now- 9990747758 Email- info@sqccertification.com Address- 2nd Floor, B, 205, 158, B Block, Sector 63, Noida, Uttar Pradesh 201301 #iso #isoindia #isostandard #sqccertification #iso50001
-
Are you ready for a challenge that will test your reflexes, push your limits, and leave you craving just one more run? Then prepare to dive headfirst into the mesmerizing world of Slope Game, a deceptively simple yet incredibly addictive online game that has captured the hearts (and frustrated the thumbs) of players worldwide. Forget elaborate storylines and complex controls. Slope Game strips gaming down to its core: pure, unadulterated skill. But don't let its minimalist aesthetic fool you. This game is a relentless test of precision and timing, offering a unique blend of frustration and satisfaction that will keep you coming back for more. So, buckle up, because we're about to take a deep dive into the world of Slope Game, exploring its origins, unraveling its mechanics, sharing pro tips, and ultimately deciding if this free-to-play phenomenon deserves a place in your gaming rotation. What Exactly Is Slope Game? Slope Game is a 3D endless runner game where you control a ball hurtling down a series of randomly generated slopes. Sounds simple, right? Wrong! The slopes are riddled with red blocks, and touching one means instant game over. Your goal is to navigate this treacherous landscape for as long as possible, racking up points and proving your mastery over the ever-increasing speed and challenging obstacles. Think of it as a modern, minimalist take on classic arcade games, where high scores reign supreme and bragging rights are fiercely contested. It’s the kind of game you can pick up for five minutes and end up playing for hours, chasing that elusive perfect run. The Masterminds Behind the Madness: Who Created Slope Game? Slope Game was developed by Rob Kay, an indie game developer with a knack for creating simple yet captivating games. While Slope Game is arguably his most successful creation, it reflects his overall design philosophy: focusing on core gameplay mechanics and delivering a polished, addictive experience. A Blast from the Past: When Did Slope Game First Roll Out? Slope Game officially launched in 2014. Since then, it has steadily gained popularity, becoming a staple in online gaming portals and a favorite pastime for gamers of all ages. Its enduring appeal speaks volumes about the quality of its gameplay and its ability to provide endless replayability. https://slopegamefree.io/
-
ISO/IEC 20000-1: 2018 Information Technology Service Management ISO/IEC 20000-1: 2018 Certification is the internationally recognized standard for IT Service Management Systems. It was developed by the International Organization for Standardization (ISO) in collaboration with the International Electrotechnical Commission (IEC) for organizations to plan, design, implement, operate, monitor, and continually improve IT services that align with business goals and deliver value to customers. This standard ensures that IT services are not just technically sound but also strategically aligned, cost-effective, and customer-focused. In today’s digital age, reliable and efficient IT services are the backbone of any successful business. Businesses are realizing the advantage of having an efficient IT service management system to demonstrate their commitment to quality IT service delivery. This Certification offers structure for developing, implementing, safeguarding, and continuously enhancing IT services. Key Features of ISO/IEC 20000-1: 2018 The ISO/IEC 20000-1: 2018 standard covers several core areas of IT Service Management, including service delivery, service transition, service design, and continual improvement. By focusing on these areas, businesses develop effective, customer-aligned ITSM frameworks to better control IT performance, meet strategic objectives, and boost service reliability. The ISO/IEC 20000-1: 2018 standard is built upon foundational principles that drive service excellence: Customer Satisfaction: Ensuring IT services meet or exceed customer expectations. Systematic Processes: Adopting a structured and consistent methodology for service management. Relationship Management: Actively managing connections with stakeholders, including clients, users, and suppliers. End-User Alignment: Aligning service delivery closely with end-user satisfaction. Continuous Improvement: Constantly reviewing and embracing evolution to stay ahead in the market. Benefits of ISO/IEC 20000-1 Certification Services Adopting ISO/IEC 20000-1 isn’t just a certification; it’s a strategic decision. Here’s how your business can benefit: Trust Building: Enhance Brand credibility among clients, partners, and regulatory bodies. Responsive Systems: Enabling agility and responsiveness to changing technologies and business requirements. Operational efficiency: Achieving cost optimization through better resource planning and reduced downtime. Minimizes IT disruptions: Mitigating risks by improving service reliability and reducing IT-related failures. Regulatory Compliance: Stay aligned with industry standards and global regulations. Market Growth: Providing access to larger contracts and new markets that require certified IT management systems. Who should obtain ISO/IEC 20000-1 Certification? ISO/IEC 20000-1 Certification is beneficial for organisations that are involved in delivering or managing IT services, whether internally or externally. Here’s a breakdown of who should obtain ISO/IEC 20000-1 Certification: IT Service Providers: Seeking structured, reliable service delivery methodologies. Internal IT Departments: Focused on improving internal services to align with internationally recognized ITSM standards. Managed Service Providers: Aiming for customer confidence and process maturity to build credibility. Public Sector IT Services: To ensure transparency, accountability, and regulatory compliance. Healthcare Organizations: To ensure data security and uninterrupted care. Educational Institutions: To build a strong, scalable tech environment for learning. Why choose us? Selecting the right partner for your ISO/IEC 20000-1 Certification journey is essential. At SQC Certification, we pride ourselves on being a reliable and experienced certification body dedicated to guiding organizations through every stage of the certification process. With a team of industry experts, a proven track record, and an unwavering commitment to excellence and client satisfaction, we ensure a smooth, transparent, and value-driven certification experience. Contact us Apply Now- https://forms.gle/LWSsNAqyDbW38eU76 Visit our website- www.sqccertification.com Call us now- 9990747758 Email- info@sqccertification.com Address- 2nd Floor, B, 205, 158, B Block, Sector 63, Noida, Uttar Pradesh 201301
-
Genius Edusoft offers an advanced School Management System developed to streamline school administration. The School Management Software as well as School ERP efficiently manage attendance, admissions exam results, and other reporting. The system also functions as an Institute Management System as well as a Campus Management System providing smooth and efficient administration to all educational institutions. Awarded the title of Best School Management Software the program includes a dedicated School Fee Collection Software that makes it easier to pay and accounting with complete accessibility and transparency. If you visit https://www.geniusedusoft.com/ the site you can gain a little knowledge of School Management System faster.
-
New Insights Into the Pathogenesis and Diagnosis of Rheumatoid Arthritis The hallmark of rheumatoid arthritis (RA) is erosive arthritis, an autoimmune disease that ultimately results in joint deformities and functional loss. It can also be complicated by pulmonary disease, cardiovascular disease, malignant tumors, and depression. The etiology of RA remains unclear. However, infections have been suggested as environmental triggers in as many as 20% of patients. Due to its perplexing etiology, a more detailed exploration of the pathogenesis of RA has been presented in an article titled "Altered antibody response to Epstein-Barr virus in patients with rheumatoid arthritis and healthy subjects predisposed to the disease" published in Immunol. The article delves deeper into the potential connection between Epstein-Barr virus (EBV) and RA, employing dependable tests that quantify antibodies directed against specific EBV antigens. So why did the research team link EBV to the development of RA? A disease similar to RA called polyarticular arthritis is induced by various viral infections, including rubella, HTLV-1, parvovirus B19, etc. Given that EBV has been connected with other autoimmune diseases such as multiple sclerosis and systemic lupus erythematosus, it is reasonable to assume that this virus may also be related to the pathogenesis of RA. Therefore, this article investigates the EBV antibody patterns in rheumatoid arthritis patients to assess the heritability of the antibody responses to the EBV-encoded EBNA1 protein, ultimately concluding that the levels of EBNA1 antibodies are notably dissimilar in RA patients compared to healthy individuals. Nevertheless, the findings reached in this article represent just a fraction of the complex investigation into the etiology of RA. Undoubtedly, the uncertain underlying causes of RA pose challenges for accurate diagnosis. RA can affect individuals of any age, but it is most frequently diagnosed in individuals between the ages of 35 and 50. Early diagnosis of RA can help identify people at risk of RA and prevent complications and disease progression. Modern imaging techniques, such as X-rays, magnetic resonance imaging, and ultrasound, aid in diagnosing RA by capturing images of affected joints. However, these methods are challenging for early RA diagnosis due to the similarity of early symptoms with those of other diseases. Additionally, detection methods that use serum markers, such as the anti-cyclic citrullinated peptide test in combination with rheumatoid factor, can improve the final diagnosis of patients with negative results from routine tests. As an efficient and precise method, IVD immunological assays and test kits rely on the specific recognition between one or more antibodies and an antigen, allowing for the detection and quantification of various antibodies in different types of samples (including serum, urine, saliva, environmental media, and more). Specifically, some rheumatoid arthritis biomarkers that have been developed for early diagnosis of RA include but are not limited to UH-RA 1, UH-RA 9, UH-RA 14, UH-RA 21, Rheumatoid Factor, 14-3-3 Eta Protein, PAD4, etc. Not only are RA biomarkers evolving, but so are their development solutions in the following approaches: * IVD Antibody Development * Antibody Pair Development * Antibody & Protein Conjugation * IVD Immunoassay Development https://www.creative-biolabs.com/drug-discovery/diagnostics/biomarker-and-antibody-development-for-rheumatoid-arthritis.htm