529 results found | searching for "investors"
-
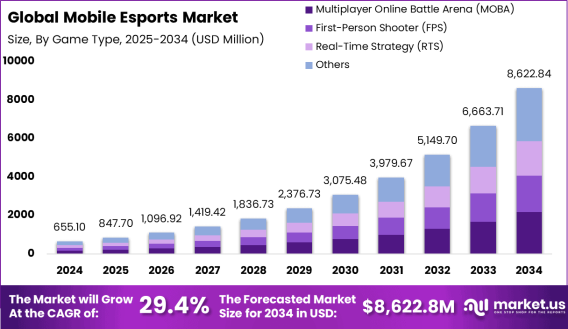
Mobile Esports Market size is growing at a CAGR of 29.4% The Global Mobile Esports Market size is expected to be worth around USD 8,622.84 Million By 2034, from USD 655.1 Million in 2024, growing at a CAGR of 29.4% during the forecast period from 2025 to 2034. In 2024, North America held a dominant market position, capturing more than a 30.4% share, holding USD 199.15 Billion revenue. Read more - https://market.us/report/mobile-esports-market/ The mobile esports market refers to the ecosystem of competitive gaming specifically designed for smartphones and tablets. It includes professional tournaments, live streaming, sponsorships, game publishers, and global communities built around mobile-based multiplayer games. With titles like PUBG Mobile, Mobile Legends, and Free Fire, mobile esports has become a cultural force, especially in regions where console and PC gaming are less accessible. The mobile esports market is growing rapidly, driven by rising smartphone penetration, affordable data plans, and widespread youth engagement. Emerging markets in Asia, Latin America, and the Middle East are fueling much of this growth. Brands, investors, and publishers are actively entering the space to tap into its vast user base and low barrier to entry. The rise in prize pools, franchising models, and mobile-first tournament platforms is transforming it into a sustainable industry. What’s really pushing this market forward is the growing demand for on-the-go gaming, shorter match formats, and inclusive digital competitions. Audiences are young, highly engaged, and hungry for entertainment that is accessible and interactive. Mobile esports gives players the thrill of real-time strategy and teamwork, all within the convenience of a handheld device.
-
Real Estate Valuation Georgia Think you know what your Georgia property is worth? Think again. Real estate valuation Georgia isn’t guesswork — it’s expert strategy. Global Valuation and Consulting LLC delivers bulletproof reports backed by deep research so investors, banks, and developers can act with confidence and clarity. Read more: https://www.globalvaluation.com/geographic-coverage/georgia-appraisals-market-feasibility-services/
-
Looking for Cross-Entity AIF Registration Consultant? Have multiple promoters or co-investors? ASC Group manages structures as your AIF registration consultant. Email info@ascgroup.in or call +91-9999043311. For more:- https://www.ascgroup.in/taxation-regulatory-services/regulatory-services/aif-registration-sebi-india #MultiEntityFunds #ASCGroup #AIFRegistrationConsultant #BusinessSafe
-
Explore a platform to buy, sell, or rent properties nationwide, featuring verified listings for apartments, plots, and PGs in cities like Noida and Hyderabad. With no extra fees, connect directly with owners, accessing detailed photos and locality insights. Ideal for students, professionals, and investors, it offers options near universities, IT hubs, and transport links. Use smart filters to find tailored properties, from budget PGs to spacious homes, with seamless communication tools. Available on mobile devices, this resource ensures a safe, efficient search, helping you navigate the real estate market with ease and confidence, no matter your location or budget.
-
Business Set Up Services in UAE offer end-to-end support for entrepreneurs and investors looking to establish a company in the region. From selecting the right jurisdiction—Mainland, Free Zone, or Offshore—to handling legal documentation, trade license acquisition, visa processing, and office setup, these services simplify the entire process. Experts ensure compliance with UAE’s laws and help avoid unnecessary delays or penalties. Whether you're a startup founder or an international investor, Business Set Up Services in UAE provide the strategic guidance and operational support needed for a smooth and successful business launch. Start your business journey in the UAE with confidence and ease. https://www.xactauditing.ae/business-set-up-services-in-uae/
-
One of the Largest Private Equity Firms in Asia – Quadria Capital Quadria Capital has emerged as one of the largest private equity firms in Asia, with a sharp focus on driving growth and impact across the healthcare and life sciences sectors. With a disciplined investment approach and deep regional expertise, we partner with businesses that deliver scalable, sustainable solutions to some of Asia’s most pressing challenges. Our mission is to transform healthcare access, affordability, and innovation—while generating strong returns for our investors. Partner with Quadria Capital – where capital meets care, and growth meets impact. Visit Us: https://quadriacapital.com/
-
Bangalore isn't just known for its IT hubs and startups — it's also a melting pot of passionate entrepreneurs, innovators, and investors. Business networking events in Bangalore provide a thriving space where professionals can share insights, explore collaborations, and learn from each other's journeys. Whether you're a new entrepreneur or a seasoned business leader, the value of face-to-face conversations and genuine connections is irreplaceable. https://livepositively.com/network-like-a-pro-business-meetups-in-bangalore/
-
Opportunities in Equity Investment in India – Quadria Capital India's dynamic and rapidly growing economy presents immense potential for strategic equity investment in India. At Quadria Capital, we specialize in identifying and nurturing high-growth opportunities across India's healthcare and life sciences sectors, creating long-term value for both investors and businesses. We work closely with portfolio companies to unlock potential, scale innovation, and drive positive change across India’s healthcare ecosystem. Visit Us: https://quadriacapital.com/approach
-
Private Equity Investment in India – Unlocking Growth with Quadria Capital India has become a hotspot for Private Equity Investment in India, attracting global investors with its rapidly growing economy, expanding middle class, and strong entrepreneurial landscape. Our strategic presence in India allows us to partner with forward-thinking businesses, providing not only capital but also deep industry expertise and operational support. Reforms and government support enhance ease of doing business and investor confidence. We invite you to join us in shaping India’s future through responsible and impactful private equity investments. Visit Us: https://quadriacapital.com/approach
-
ASC Group assists growing businesses in raising funds via SME IPOs. Their team ensures SEBI compliance, handles disclosures, and helps you attract the right investors. For more:- https://www.ascgroup.in/sme-ipo-bse-listing-consultant-advisory-readiness-service-firm-india/