73 results found | searching for "vaccine"
-
The global Bioprocess Bags Market was valued at USD 3.50 billion in 2023 and is projected to reach USD 13.78 billion by 2032, growing at an impressive CAGR of 16.46% between 2024 and 2032. This remarkable growth reflects the surging adoption of single-use technologies across the biopharmaceutical sector, where efficiency, scalability, and contamination control have become essential for both research and large-scale drug production. Bioprocess bags are critical tools for storage, mixing, and transport of biopharmaceutical fluids, offering superior sterility and reducing the risks associated with traditional stainless-steel systems. Their increasing integration into upstream and downstream processing highlights a transformative shift in the way biologics, vaccines, and cell-based therapies are developed and manufactured. Market Dynamics: Why the Industry Is Scaling at Unprecedented Levels The rapid expansion of biologics and biosimilars pipelines worldwide is a major force fueling the demand for bioprocess bags. As pharmaceutical companies face heightened pressure to bring therapies to market quickly, flexible and reliable solutions like bioprocess bags offer significant advantages. They lower capital costs, reduce cleaning validation requirements, and minimize the risk of cross-contamination. Furthermore, the COVID-19 pandemic accelerated the acceptance of single-use technologies. Manufacturers worldwide experienced firsthand the flexibility these systems provided in ramping up vaccine production. That momentum continues to shape bioprocessing strategies, with companies investing heavily in disposable solutions to ensure agility and speed. Bioprocess bags are not just limited to large-scale manufacturing. They are increasingly being adopted in academic research labs, contract development and manufacturing organizations (CDMOs), and emerging biotech startups, where scalability and cost-effectiveness are equally critical. Technological Advancements Enhancing Market Potential The bioprocess bags industry is witnessing significant innovation in materials, design, and performance. Leading manufacturers are focusing on developing multilayer films that provide enhanced durability, high oxygen barrier properties, and compatibility with a wide range of biologic materials. Additionally, bags are being designed with advanced monitoring systems that integrate sensors to track pH, dissolved oxygen, and other key parameters in real time. These smart bag solutions align with the biopharma industry’s push toward process intensification and continuous manufacturing. The development of customizable and scalable bag formats is further supporting small- and mid-sized biotech firms that require flexibility without compromising compliance with regulatory standards. Regional Outlook: North America and Asia-Pacific at the Forefront North America continues to lead the bioprocess bags market, driven by the strong presence of biopharmaceutical giants, advanced research infrastructure, and favorable regulatory frameworks. The region’s focus on biologics, particularly monoclonal antibodies and gene therapies, sustains robust demand for single-use solutions. Meanwhile, Asia-Pacific is emerging as a hotspot for growth. Rapidly expanding biotech clusters in countries like China, India, and South Korea are attracting global investments. Governments in the region are promoting domestic biologics production, further boosting adoption of bioprocess bags. The lower cost of production combined with strong demand for biosimilars positions Asia-Pacific as a key growth engine for the forecast period. Key Market Drivers Booming Biologics and Biosimilars Market: Rising prevalence of chronic diseases and demand for advanced therapies have placed biologics at the center of global healthcare, propelling the need for reliable bioprocessing solutions. Shift Toward Single-Use Technologies: The move away from stainless-steel systems to disposable bags significantly reduces downtime, contamination risks, and operational costs. Growing Investment in Cell and Gene Therapy: Breakthroughs in regenerative medicine demand flexible and sterile solutions that bioprocess bags are uniquely designed to provide. Rapid Expansion of Contract Manufacturing Organizations: As CDMOs scale operations globally, the reliance on single-use technologies becomes indispensable. Competitive Landscape The bioprocess bags market is highly competitive with a mix of global leaders and specialized niche players. Companies are focusing on collaborations, acquisitions, and product launches to strengthen their portfolios. Leading players are also investing in expanding production capacities to meet the surging global demand. Recent trends show a rise in partnerships between suppliers and CDMOs to co-develop customized solutions. Such collaborations enable end-users to achieve process efficiencies while ensuring compliance with regulatory standards. Challenges to Watch Despite its strong trajectory, the market does face hurdles. Concerns related to leachables and extractables from plastic materials remain under scrutiny, particularly from regulatory authorities. Additionally, supply chain disruptions for raw materials can pose risks to production continuity. However, industry stakeholders are addressing these challenges through rigorous testing protocols, improved material science, and diversification of supply chains to ensure consistent availability of high-quality bioprocess bags. Future Outlook The bioprocess bags market is positioned for exceptional growth throughout the next decade. The convergence of biologics expansion, single-use adoption, and smart technology integration sets the stage for continued innovation. With advancements in material engineering and automation, bioprocess bags are expected to evolve from simple storage tools to highly sophisticated components that actively support biomanufacturing. As the healthcare industry shifts toward precision medicine, biologics and cell-based therapies will demand even greater flexibility and sterility in manufacturing processes. Bioprocess bags are uniquely aligned to meet these evolving needs, cementing their role as a cornerstone of modern bioprocessing. Industry analysts predict that the next phase of growth will be marked by hybrid systems, where single-use technologies like bioprocess bags coexist with stainless-steel infrastructure to optimize performance, scalability, and sustainability. Conclusion With a projected market value of USD 13.78 billion by 2032, the bioprocess bags industry is set to reshape the global biopharmaceutical manufacturing landscape. Its rapid adoption underscores the industry’s commitment to efficiency, sterility, and adaptability in an increasingly competitive market environment. For stakeholders across the value chain, from biotech startups to global pharmaceutical leaders, investing in bioprocess bag solutions represents not just an operational advantage but a strategic imperative. Read More: https://www.snsinsider.com/reports/bioprocess-bags-market-6853
-
Real-time Monitoring Solutions For Cold Chain Market size is growing at a CAGR of 22.50% The Global Real-time Monitoring Solutions For Cold Chain Market size is expected to be worth around USD 116.8 Billion By 2034, from USD 15.35 Billion in 2024, growing at a CAGR of 22.50% during the forecast period from 2025 to 2034. North America led the global market in 2024, accounting for more than 35.4% of the share and generating roughly USD 5.4 billion in revenue. Read more - https://market.us/report/real-time-monitoring-solutions-for-cold-chain-market/ Real-time monitoring solutions for the cold chain market refer to advanced systems designed to track and manage temperature-sensitive products, such as pharmaceuticals, food, and chemicals, throughout their journey from production to delivery. These solutions use technologies like IoT sensors, RFID tags, and cloud-based platforms to provide continuous, live data on temperature, humidity, and other environmental conditions. By ensuring products remain within specified parameters, these systems prevent spoilage, maintain quality, and ensure compliance with safety standards. The focus is on creating a seamless, transparent supply chain where stakeholders can access real-time insights to make quick, informed decisions, reducing risks and enhancing efficiency. The real-time monitoring solutions for the cold chain market is a rapidly growing sector, valued at around USD 12.4 billion in 2023 and projected to expand at a CAGR of over 23% through 2030. This market encompasses hardware like sensors and data loggers, software for analytics, and services for implementation and support. It serves industries like pharmaceuticals, food and beverages, and logistics, with North America leading due to its robust infrastructure and Asia-Pacific showing the fastest growth due to rising demand for perishable goods. The market’s expansion is fueled by the need for transparency, regulatory compliance, and the global rise in e-commerce, particularly for temperature-sensitive products. Top Driving Factors: The growth of this market is driven by the surging demand for temperature-sensitive products like vaccines, biologics, and fresh foods, which require precise conditions to maintain efficacy and safety. Stringent regulations from bodies like the FDA and EMA push companies to adopt reliable monitoring systems to avoid costly penalties. The rise of e-commerce, especially online grocery and pharmaceutical deliveries, demands robust cold chain solutions to ensure doorstep quality. Additionally, global trade expansion and consumer expectations for high-quality, fresh products are compelling businesses to invest in real-time monitoring to safeguard their supply chains. Demand Analysis: Demand is skyrocketing, particularly in pharmaceuticals and food sectors, where product integrity is non-negotiable. The pharmaceutical industry, spurred by the need for vaccine distribution post-COVID, accounts for a significant share, with biologics and specialty drugs requiring strict temperature control. In food and beverages, the push for organic and minimally processed items, coupled with e-commerce growth, drives demand for monitoring solutions. Emerging markets like India and China are seeing rapid uptake due to urbanization and rising disposable incomes, increasing the need for cold chain infrastructure to support perishable goods.
-
JYNNEOS, also referred to as Imvamune or Imvanex in some regions, is a non-replicating, live-attenuated vaccine derived from the Modified Vaccinia Ankara (MVA) strain. It is designed for use against both smallpox and mpox. Learn more, https://monkeypox.creative-biolabs.com/what-is-mpox-vaccine.htm.
-
Staying informed about vaccine code changes for the 2024–2025 period is essential for accurate medical billing and compliance. https://www.outsourcestrategies.com/blog/ama-announces-changes-vaccine-codes-2025/
-
Respiratory Syncytial Virus Vaccine & Antibody Pipeline Market The latest report by Precision Business Insights, titled respiratory syncytial virus vaccine and antibody pipeline market covers complete information on market size, share, growth, trends, segment analysis, key players, drivers, and restraints.
-
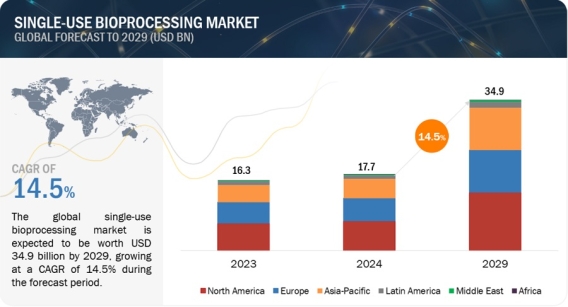
Global Single Use Bioprocessing Market Worth $34.9 Billion by 2029: Key Insights and Analysis https://www.marketsandmarkets.com/Market-Reports/single-use-bioprocessing-market-231651297.html The single-use bioprocessing market is expected to grow at a CAGR of 14.5% during the forecast period. The market is estimated to be around USD 17.7 billion in 2024 and is projected to reach USD 34.9 billion by 2029. Growing biologics & biosimilars market, need for low capital investment, a surge in single-use bioprocessing adoption among CDMOs & CMOs, and advantages such as increased productivity & reduced risk of cross contamination, are some factors responsible for the growth of the market. On the other hand, issues related to leachables & extractables, regulatory compliance, and discussions about PFAS restrictions are hampering the market growth. Download a PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=231651297 Based on products, the single-use bioprocessing market is segmented into equipment and consumables. The consumables segment accounted for the largest share and is also estimated to grow at the highest CAGR of the single-use bioprocessing market. This can be attributed to their repeated purchase, higher usage, frequent replacement, and broad application range. Based on application, the single-use bioprocessing market is segmented into cell culture, filtration, purification, mixing, storage & transfer, and other applications. The filtration segment accounted for the largest share of the single-use bioprocessing market. The large share of this segment can be attributed to the wide use of equipment for various filtration applications in the pharmaceutical industry, increased filtration requirements in the biopharmaceutical industry, and the higher requirement for these filters compared to consumables and filtration accessories. Based on workflow, the single-use bioprocessing market is segmented into upstream and downstream bioprocessing. The growth of the single-use bioprocessing market for upstream bioprocesses is driven by technological advancements and strategic collaborations between industry players. For instance, in June 2023, Culture Biosciences and Cytiva collaborated to introduce new bioprocess solutions that leverage digitization, in-silico approaches, and virtual monitoring and control of experiments. This collaboration revolutionized upstream processing by providing customers unprecedented access to cutting-edge technologies and methodologies, fueling the demand for advanced bioprocessing solutions. Based on molecule type, the single-use bioprocessing market is segmented into monoclonal antibodies (mAbs), vaccines, therapeutic proteins & peptides, and cell & gene therapies. mAbs accounted for the largest share of the single-use bioprocessing market. The large share is attributed to the increasing pharmaceutical R&D drug pipeline, increasing incidence of cancer, and rising usage & demand for cancer therapy. Based on the end user, the single-use bioprocessing market is segmented into pharmaceutical & biotechnology companies, academic & research institutes, and CMOs & CROs. In 2023, pharmaceutical & biotechnology companies accounted for the largest share of the single-use bioprocessing market due to their higher adoption of single-use bioprocessing equipment and consumables, the stringent regulatory scenario, increased R&D activities, increased production of biologics, and rising manufacturing of biologics and biosimilars in developed countries. Based on region, single-use bioprocessing market is segmented into six major regions including North America, Europe, Asia Pacific, Latin America, Middle East, and Africa. In 2023, North America dominated the single-use bioprocessing market. Europe held the second largest share followed by the Asia Pacific. The Asia Pacific market is expected to show the highest growth rate during the forecast period. Growing pharmaceutical R&D activities, increasing demand for biosimilars, significant investments by biopharmaceutical companies & CMOs in emerging Asia Pacific countries, are some of the factors contributing to the regional growth. Key players in the single-use bioprocessing market include Sartorius AG (Germany), Danaher Corporation (US), Thermo Fisher Scientific, Inc. (US), Merck KGaA (Germany), Avantor, Inc. (US), 3M Company (US), Repligen Corporation (US), Entegris (US), Getinge AB (Sweden), Parker Hannifin Corporation (US), Alfa Laval Corporate AB (Sweden), Saint-Gobain (France), Eppendorf SE (Germany), Corning Incorporated (US), Eaton Corporation plc (Ireland)v, METTLER TOLEDO (US), Porvair PLC (UK), Lonza (Switzerland), ABEC, INC. (US), NewAge Industries (US), PBS Biotech, Inc. (US), Sentinel Process Systems, Inc. (US), Meissner Filtration Products, Inc. (US), SATAKE MultiMix Corporation (Japan), Hamilton Company (US), Membrane Solutions (US), Cole-Parmer Instrument Company, LLC (US), Distek, Inc. (US), Esco Lifesciences Group Ltd. (US), and TECNIC Bioprocess solutions (Spain). Recent Developments of the Single Use Bioprocessing Industry: In April 2024, Cytiva introduced the Xcellerex magnetic mixer, a single-use mixing system in 2,000 and 3,000 L capacities for large-scale mAb, vaccine, and genomic medicine manufacturing processes. In April 2023, Merck KGaA launched the Ultimus single-use process container film designed to provide extreme durability and leak resistance for single-use assemblies used for bioprocessing liquid applications.
-
Breakthrough mRNA Research Garners 2023 Nobel Prize in Physiology or Medicine On October 2nd, the Nobel Assembly unveiled the recipients of the 2023 Nobel Prize in Physiology or Medicine: scientists Katalin Karikó and Drew Weissman. Their pioneering research in messenger ribonucleic acid (mRNA) has reshaped vaccine development, notably amid the COVID-19 pandemic. Their work has not only saved countless lives but has also alleviated the severity of cases, relieving pressure on healthcare systems and facilitating the global reopening of societies. To date, mRNA vaccines, administered over 13 billion times worldwide, have played a pivotal role in fighting the pandemic. Scientists have delved into mRNA's potential for vaccine development since the 1990s. The laureates' work "revolutionized our comprehension of mRNA's interaction with the immune system," crucial in the swift creation of mRNA vaccines for SARS-CoV-2 during the ongoing global health crisis. These vaccines deliver the spike protein mRNA sequence into cells using lipid nanoparticles (LNPs) as carriers. This innovative method triggers protein production, activating immune cells and eliciting responses like the creation of neutralizing antibodies and antigen-specific T cells. mRNA-based SARS-CoV-2 vaccines boast rapid production and cost-effectiveness. By amplifying antigens through mRNA synthesis, high concentrations of neutralizing antibodies are achieved, enhancing vaccine efficacy. In contrast, producing vaccines based on whole viruses or viral proteins necessitates extensive cell cultures, complicating rapid pandemic vaccine production. SARS-CoV-2 vaccine candidates underwent testing in animal models like ACE2 humanized mice, ferrets, and rhesus macaques. Exogenous mRNA corresponding to viral gene fragments enables host cells to produce viral proteins, stimulating immune responses and serving as vaccine candidates. Yet, extracellular mRNA production suffers from instability and inefficient delivery. The laureates' research showcased that modifying extracellular mRNA's nucleotide bases could make the host "recognize" exogenous mRNA as self-mRNA. This modification reduces inflammatory reactions and boosts protein production after delivery, removing key hurdles in mRNA's clinical application. This breakthrough paves the way for agile mRNA vaccine development for infectious diseases and holds potential for delivering therapeutic proteins and treating specific cancer types However, mRNA is inherently unstable and prone to enzymatic degradation within the body. Another challenge lies in the potential for mRNA to trigger intense inflammatory responses, potentially harming cells and tissues. Despite skepticism and rejection, Karikó and Weissman persevered. In 2005, they published a groundbreaking paper addressing these challenges. By modifying mRNA's building blocks, nucleotides, they enhanced stability and reduced immunogenicity. Additionally, they devised a method employing lipid nanoparticles to deliver mRNA into cells, safeguarding and transporting mRNA within minuscule lipid bubbles. Karikó and Weissman's work stands as a groundbreaking transformation in anti-SARS-CoV-2 candidates and public health, illustrating the potency of curiosity-driven science and resilience. Their achievements inspire researchers and innovators worldwide to explore mRNA technology's potential in enhancing human health and well-being. https://sars-cov-2.creative-biolabs.com/mrna-vaccine-development-services-for-sars-cov2.htm
-
Cancer vaccines consist of one or more antigenic components, including live-attenuated or killed viral or bacterial particles, proteins, polysaccharides, polynucleotides, and particle conjugates. Additionally, other excipients like adjuvants may be present. Not only the chemical and structural integrity of various components must be maintained, but the immunogenicity must be ensured. Thus, the complexity of vaccines creates a unique challenge for characterization. Learn more about GMP cancer vaccine analysis: https://www.creative-biolabs.com/car-t/gmp-cancer-vaccine-analysis-and-qualification.htm