39 results found | searching for "viability"
-
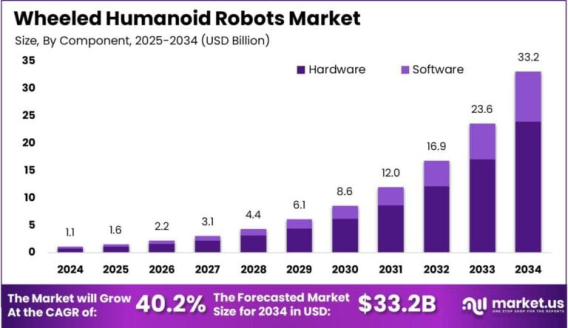
Wheeled Humanoid Robots Market size is growing at a CAGR of 40.20% The Global Wheeled Humanoid Robots Market size is expected to be worth around USD 33.2 Billion By 2034, from USD 1.13 Billion in 2024, growing at a CAGR of 40.20% during the forecast period from 2025 to 2034. In 2024, North America led the wheeled humanoid robots market with a 43.2% share and USD 0.4 billion in revenues. The U.S. market was valued at USD 0.44 billion and is growing rapidly, with an estimated CAGR of 38.7%. Read more - https://market.us/report/wheeled-humanoid-robots-market/ Wheeled humanoid robots are advanced robotic systems designed with a humanoid body structure but move using wheels instead of legs. These robots are typically developed for applications requiring both human-like interaction and efficient locomotion in indoor and semi-structured environments. Their ability to communicate, perform service tasks, and navigate smoothly makes them suitable for customer service, healthcare, logistics, and personal assistance. Their wheeled design simplifies balance and speed control, making them more practical than bipedal robots in several use cases. The wheeled humanoid robots market is witnessing steady growth, driven by rising demand in sectors like retail, hospitality, and eldercare. With industries increasingly looking to automate tasks involving human interaction, the market is expected to grow as both startups and major players invest in robotic solutions. Asia-Pacific and North America remain key regions due to their strong robotics manufacturing base and growing consumer service sectors. Government-led tech initiatives and private sector R&D efforts are also accelerating development and deployment across industries. One of the main driving forces is the rapid evolution of AI and machine learning, which enables these robots to interpret human behavior and adapt accordingly. Growth in urban automation, labor shortages in service sectors, and the need for contactless operations post-pandemic are further fueling market adoption. As people become more comfortable with service robotics in everyday settings, the market is benefitting from increased public acceptance and commercial viability.
-
Cytotoxicity Assays are vital in evaluating the toxic effects of substances like drug candidates or biomaterials on living cells. These assays, including methods like Trypan Blue, MTT, and Alamar Blue, help measure cell viability and cytotoxicity in vitro. They are widely used in drug discovery, safety assessment, cancer research, and environmental studies, offering rapid, cost-effective, and animal-free testing solutions. Kosheeka provides high-quality cells optimized for reliable and high-throughput cytotoxicity assays. Read more... https://www.kosheeka.com/in-vitro-cytotoxicity-assays-applications-in-drug-discovery/
-
The human peripheral blood-derived mononuclear cells are obtained from peripheral blood, an easily available, non-invasive alternative source to bone marrow. A pool of mononuclear cells is a mixed population primarily comprised of immune cells like T cells, B Cells, Monocytes, endothelial cells, fibroblasts, platelets, and very small embryonic-like stem cells as well. Studies have also confirmed the presence of 0.1% Human Peripheral Blood-Derived Mesenchymal Stem cells in a fraction of mononuclear cells. Such a pool of important cells can serve as a powerful tool for in vitro research studies including but not limited to the: Wound healing, and tissue repair Immunotherapies and regulation of inflammatory conditions - Exosomes derived from a fraction can be a great tool to explore communication pathways associated with blood cancers. In vitro models for drug testing and drug discovery Why should you choose Kosheeka for your requirement of Human Peripheral Blood-Derived Mononuclear Cells? Isolated from peripheral blood of varied donor databases with a pool of male and female donors as well. Highly characterized and well counted for viability, surface marker analysis Suitable for studying co-cultures with a variety of cell types including endothelial cells Are extensively used in pre-clinical setups For more information about the product availability, please feel free to contact us at +91-9654321400.
-
Has your doctor brought up Erectile Dysfunction Stem Cell Treatment yet? Are you on the hunt for the best stem cell treatment for erectile dysfunction near Noida, huh? Fret not, because Advancells has got you covered! We are a leading name in the cell manufacturing biosphere with our in-house GMP-compliant laboratory, where we produce our cells. It means that we have placed strict quality checks in place to ensure that you get the best quality stem cells that are viable. All that means is that these high-quality cells will translate into successful treatments for those who need them. It is working towards helping you gain back your confidence. Here is why Advancells is the perfect partner in your recovery journey: • We ensure that you understand from where these cells are isolated. • We do rigorous testing of cells for viability and quality. • The stem cells are gonna go straight to the source of the issue instead of just dealing with the surface stuff. https://www.advancells.com/stem-cell-treatment-erectile-dysfunction/
-
Podiatry clinics face specific medical billing issues due to the challenges of categorizing various foot-related illnesses and different insurance coverage. Leveraging professional medical billing services can help providers can maintain their financial viability by streamlining claim submission, lowering risk of claim denials, and increasing revenue. https://www.outsourcestrategies.com/medical-billing/
-
At Creative Biolabs, we understand that every neuroscience inquiry is unique. Our dedicated team collaborates closely with researchers to design customized neural cell assays tailored to their specific questions. This ensures that the assays precisely target the neural cell types and functional aspects of interest, maximizing the quality and relevance of the data obtained. https://neuros.creative-biolabs.com/cell-viability-and-cytotoxicity-assay.htm
-
Neuroinflammation can be assessed by evaluating different aspects of microglia and astrocytes in the central nervous system (CNS). Some key assays include glial activation, proinflammatory cytokine concentrations, blood-brain barrier permeability, and neuronal viability. CNS injury, brain infection, toxins, autoimmune diseases, and other conditions may all lead to the aberrant release of inflammatory factors. Neuroinflammation is a potential mechanism underlying Alzheimer's disease and other neurological disorders. Creative Biolabs' neuroinflammation assays are efficient and accurate scientific tools that can assist researchers in understanding the biology of neurological disorders and screening prospective lead candidates. https://neuros.creative-biolabs.com/neuroinflammation-assay.htm
-
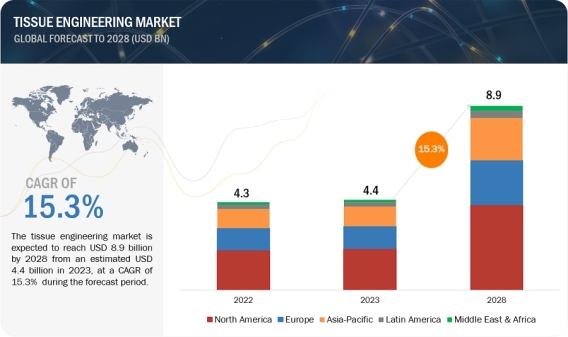
Driving Regenerative Medicine Advances: Tissue Engineering Market to Reach $8.9 Billion by 2028 https://www.marketsandmarkets.com/Market-Reports/tissue-engineering-market-34135173.html The tissue engineering market is expected to reach USD 8.9 billion by 2028 from an estimated USD 4.4 billion in 2023, at a CAGR of 15.3% during the forecast period. The growth is attributed to numerous factors including increasing incidences of road accidents, rising demand for regenerative medicines for the treatment of chronic diseases, and growing technological advancements in the biopharmaceutical sector. In addition, increasing research activities in the field of tissue engineering is promoting the growth of the tissue engineering market. Download a PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=34135173 3D bioprinting is an innovative technology in tissue engineering, that enables the precise fabrication of intricate tissue structures, vital for regenerative medicine and drug testing. Its development is primarily propelled by continual technological advancements, including improved bio inks and printing techniques, coupled with the critical need to address organ shortages and revolutionize medical treatments. Material innovation also plays a pivotal role, in fostering the creation of biocompatible substances essential for enhancing the viability and functionality of bio-printed tissues. Additionally, collaborative research efforts among multidisciplinary fields drive progress, promising transformative solutions in healthcare. Based on product type, the tissue engineering market has been segmented into scaffolds, tissue grafts, and other products. In 2022, scaffolds accounted for the largest market share. Scaffold-driven tissue engineering hinges on biocompatibility, mechanical robustness, and optimal pore structure for cell infiltration and nutrient exchange. Integration of bioactive cues and vascular-like properties also fuels advancements, crucial for crafting functional and viable engineered tissues. These driving factors collectively shape scaffold design, influencing their efficacy in tissue engineering applications. Based on material, the global tissue engineering market is segmented into synthetic materials and biologically derived materials. Synthetic material accounted for major market share of the tissue engineering market in 2022. The large share of this segment can be attributed to the cost effectiveness of the synthetic products as compared to the biologically derived products. In addition, increasing demand for advanced and cost-effective tissue engineering solutions is propelling the growth of the synthetic material segment in the tissue engineering market. Based on application, the tissue engineering market is segmented into orthopedics & musculoskeletal disorders, dermatology & wound care, dental disorders, cardiovascular diseases, and others. In 2022, orthopedics & musculoskeletal disorders accounted for the largest share of the global tissue engineering market. The large share of the segment can primarily be attributed to rising demand for high quality tissue engineering products for the repair and reconstruction of damaged tissues or organs. Additionally, increasing incidences of road accidents are promoting the adoption of tissue engineering products for tissue reconstruction. Based on end users, the tissue engineering market has been segmented into hospitals, specialty centers and clinics, and ambulatory surgical centers. Hospitals accounted for the largest market share of the tissue engineering market. The large share of the segment can primarily be attributed to the increasing demand for advanced regeneration solutions for the reconstruction and regeneration of tissues. Additionally, the increasing rate of chronic and degenerative diseases is driving the demand for hospitals. The key regional markets for the global tissue engineering market are Europe, North America, Asia Pacific, Latin America, and the Middle East & Africa. In 2022, North America accounted for the largest share of the market. The large share of this region can be attributed to the growing technological developments in the healthcare sector and rising research activities for the advancements of the tissue engineering field. However, most of the growth in the market is expected from emerging countries across Asia Pacific. During the forecast period, the Asia Pacific is expected to grow with the fastest CAGR due to the increasing adoption of innovative technologies for the treatment of chronic and degenerative diseases. Key players in the global tissue engineering market include Organogenesis (US), AbbVie Inc. (US), Baxter (US), BD (US), B. Braun (Germany), TEIJIN Limited (Japan), Institut Straumann AG (Switzerland), Integra Lifesciences (US), Johnson & Johnson Services, Inc. (US), Medtronic (Ireland), NuVasive, Inc. (US), Stryker (US), Terumo Corporation (Japan), W. L. Gore & Associates Inc. (US), Zimmer Biomet (US), Smith & Nephew plc (UK), MIMEDX Group, Inc. (US), BioTissue (US), CollPlant Biotechnologies Ltd. (Israel), Sumitomo Pharma Co., Ltd. (Japan), Matricel GmbH (Germany), Mallinckrodt (US), Regrow Biosciences Pvt Ltd (India), Vericel Corporation (US), Tecnoss S.R.L. (Italy), Tegoscience (South Korea), and Tissue Regenix (UK). Recent Developments of Tissue Engineering Industry In September 2023, MIMEDX Group Inc. launched EPIEFFECT to broaden its advanced wound care product portfolio. EPIEFFECT is a lyophilized human placental-based allograft consisting of amnion and chorion membranes. In July 2023, Teijin Limited launched SYNFOLIUM, a cardiovascular surgical patch and received manufacturing and marketing approval in Japan. The cardiovascular surgical patch is used for the surgical treatment of congenital heart disease (CHD). In July 2021, Integra Lifesciences introduced SurgiMend, a collagen matrix. The product is used for soft tissue repair and reconstruction. In May 2020, AbbVie Inc. (US) acquired Allergan plc (Ireland) to expand the product portfolio in therapeutics categories. Allergan plc provides new growth opportunities to AbbVie Inc. in neuroscience with Vraylar, Botox therapeutics, and global aesthetics business.
-
The liver's primary functional cells are hepatocytes, crucial for liver functions. These cells can be obtained from sources each with their own advantages and disadvantages. While primary hepatocytes offer the accurate physiological representation they face challenges in terms of availability, viability and limited ability to grow. Liver cell lines provide a source but may show changes in characteristics. Hepatocytes derived from pluripotent stem cells show potential for personalized medicine but need further development. A thorough understanding of hepatocyte features is essential for progressing liver research. Learn more: https://shorturl.at/OvyfJ
-
A minimum viable product is best if you want to test the viability of your product. Here is the guide to the MVP development cost and cost optimization tips. https://ripenapps.com/blog/mvp-development-cost/ #MVP_development_cost #MVP_app_development_company #Tech_Investment #Cost_Optimization #Product_Development #Digital_Strategy #MVP_development_services